Testing ventilator and breathing assistance apparatus components


Solution
- Software-controlled tensile and compression tester
- Software-controlled torque testing system
- Appropriate grips for the test being implemented
Benefits
- Intuitive software to apply bespoke procedures or international standards
- Interchangeable grips and fixtures enabling multiple tests on one machine
- Pre-programmed test routines for QC checks at point-of-manufacture
- Extensive reporting to log results for audit purposes
Requirement
A ventilator (respirator) provides ‘mechanical ventilation’ by moving breathable air into and out of the lungs of patients unable to breathe sufficiently well unaided. Such fundamental hospital equipment is essential for all patient care where assisted breathing is necessary. This can be for any number of standard medical procedures, planned operations or A&E injury events requiring anaesthesia, as well as diagnosed or chronic conditions directly or indirectly affecting respiration. Ventilators also play a critical role in nursing homes and for many home-care services—even in ‘normal’ circumstances.
Under the exceptional circumstances of a pandemic threat, most recently COVID-19 (coronavirus), where the virus often attacks the human respiratory system directly or presents as a secondary condition, e.g., Acute Respiratory Distress Syndrome (ARDS), the demand for ventilation systems escalates. Established manufacturers of mechanical ventilators are being joined in the fight by disparate manufacturing companies from other industries, all producing ‘open-source’ ventilators (reverse-engineered breathing equipment) to cover potential supply shortages.
The modern continuous positive airway pressure (CPAP) ventilator has advanced somewhat from the historical ‘iron lung’; adding portability alongside sophisticated electronic control of its operating and monitoring functions. The main systems include:
- Power sources: electrical power, gas supply and pressure generator.
-
Gas delivery control:
- accumulator, blender and inspiratory flow regulator
- humidification equipment
- ‘patient circuit’ sub-system between mechanical power and the patient
- Expiratory pressure regulator; Positive End-Expiratory Pressure (PEEP) valve
- Monitoring: gas concentration, flow, pressure and volume sensors
- Safety features: intake particle, pre-circuit bacteria and expired gas filters; moisture/heat exchange systems and alarms.
As the ventilator is classified as a ‘life-critical’ system, extensive testing must be undertaken to ensure that they are highly reliable in performing their multiple constituent functions. Additionally, the ventilator must be easy to use by clinical staff, often in high-pressure situations wearing complex protective clothing, so its ergonomic functionality is tested.
Solution
A ventilator and its parts, including applicable accessories, must have adequate mechanical strength when subjected to mechanical stress caused by normal use, pushing, impact, dropping and rough handling.
Product/component and materials testing is, therefore, a vital part ensuring a ventilator is correctly manufactured and performs to its specification. Mecmesin has over 40 years of experience in providing force and torque testing solutions, not only to medical device manufacturers but across all engineering industries, so even Rapidly Manufactured Ventilator Systems (RMVS) producers can implement appropriate quality and durability checks.
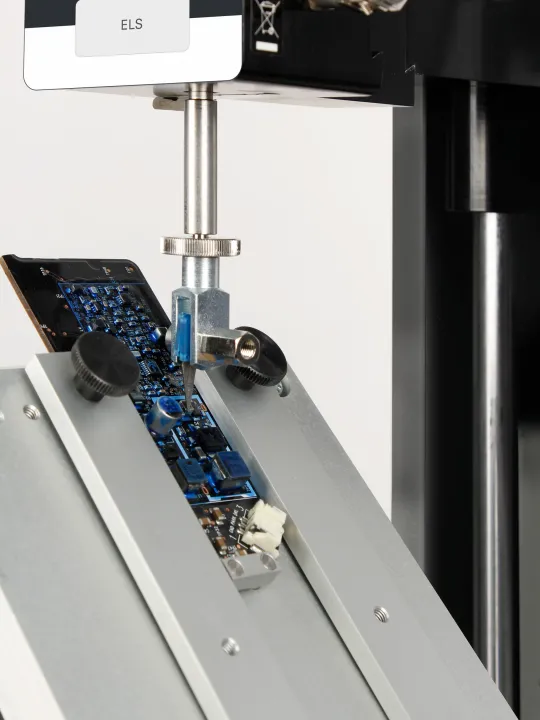
Typical force and torque tests include:
- Pumps, bearings, turbines: compression, bend strength
- Pressure regulation valves: adjustment and opening characteristics
- Spring assemblies: compression spring rate
- Touch-button screens: local mechanical strength
- Rotary control knobs: feel and engagement feedback
- PCB soldered joints: break strength
- Crimped wire terminals and connectors: pull strength
- Patient circuit tubing: physical crush resistance, elongation
- 3d-printed and other composite material components: mechanical strength
Any medical or pharma industry product (or component) must be functionally reliable in this highly regulated sector. International test standards may relate directly to the necessary performance testing that manufacturers must comply with. VectorPro software supports FDA 21 CFR compliance, its secure architecture with avalable modules to facilitate electronic records and signature audit trails.
Related case study for NIV face mask strength testing.
Test equipment
- Force, tensile, compression testers
- Torque testers
- Standard accessories for most tests, with adjustability, pneumatic actuation and quick-change features
- Mecmesin can 3D-print custom-fit fixtures for exact matching to component geometry